靶向GPCR的药物研发
一、小分子药物和抗体药物的特点和优势
G蛋白偶联受体(GPCR)是膜蛋白,在信号转导和细胞通讯中发挥着重要作用。因此,GPCR通常成为药物治疗靶点,包括小分子药物和抗体药物。靶向GPCR的小分子药物和抗体药物具有各自的特性和优势[1]。
小分子药物通常用于通过与GPCR的正构或变构结合位点结合来调节其活性。小分子药物的优点是可以口服,并且容易穿透细胞膜到达目标。小分子药物的制造成本相对较低,可以大批量生产。 然而,小分子药物通常具有脱靶效应,可能对目标受体的特异性较低,而导致副作用[2]。
相比之下,抗体药物是大型蛋白质,可以结合于GPCRs的特定表位。它们通常高度特异于靶标受体,并且可以通过改造在血液中有更长的半衰期。,抗体药物可以激活或阻断下游信号通路,并且与小分子药物相比,不容易发生非特异性作用。然而抗体药物需要通过静脉注射进入体内,这可能会限制其方便性[3]。
总体而言,小分子药物和抗体药物对GPCRs的靶向有互补的优点和局限性,如何选择取决于特定的治疗应用和目标受体的特性。
二、 GPCR药物发现过程中潜在激动剂或拮抗剂的验证方法
为了验证潜在的GPCR激动剂或拮抗剂的功效,研究人员使用各种测定方法,包括细胞实验,放射性配基结合实验和功能验证。
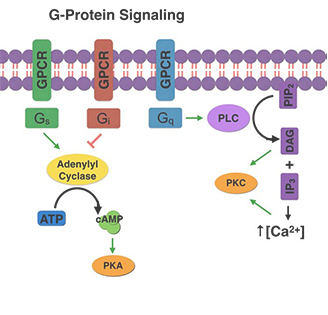
(由G蛋白介导的GPCR信号通路)
细胞实验(Cell-based assays)
细胞实验通常用于验证潜在的GPCR激动剂或拮抗剂。这些实验涉及测量细胞内信号转导途径在配体结合后的变化。可以测量的信号转导途径包括腺苷酸酰化酶(adenylyl cyclase)的激活、细胞内钙离子释放以及MAP激酶途径的激活[4]。
一些商业试剂盒可用于验证GPCR活性的细胞实验。例如,Promega提供GloSensor cAMP测定系统,允许实时监测活细胞中的cAMP水平[5]。同样,Molecular Devices的FLIPR钙浓度测定系统(FLIPR Calcium Assay)通过测量钙动员实现GPCR活性的高通量筛选[6]。
放射性配基结合实验 (Radioligand binding assays)
放射性配基结合实验是验证潜在的GPCR激动剂或拮抗剂的另一种常用方法。这种方法使用放射标记的配体测量化合物对受体的结合亲和力。放射性配基结合实验非常敏感,可以检测到即使很小的结合亲和力变化。
可用于GPCR的放射性配基结合实验的商业试剂盒有PerkinElmer的膜靶系统。该试剂盒提供了多种GPCR的预验证膜和配体,简化了测定设置并最小化了变异性。
功能验证(Functional assays)
功能验证实验用于测量 GPCR 激活的下游效应,例如离子通道的激活或细胞内第二信使的释放。 这种方法不仅可用于验证 GPCR 的潜在激动剂或拮抗剂,并且通常用于确定这些化合物的作用机制[7]。
有几种商业试剂盒可用于GPCR活性的功能测定。 例如,DiscoverX的PathHunter分析系统使用基因编码的报告系统测量下游信号通路的激活。 同样,Promega的Beta-Glo检测系统可测量 β-arrestin 的激活,β-arrestin是 GPCR 脱敏和内化的关键介质[8]。
总之,验证 GPCR 的潜在激动剂或拮抗剂是药物开发的重要一步。通过使用这些检测方法,研究人员可以鉴定出对 GPCR 具有高亲和力和特异性的化合物,从而开发出有效且安全的疗法。
G protein-coupled receptors (GPCRs) are membrane proteins that play important roles in signal transduction and cellular communication. As such, GPCRs are often targeted by therapeutics, including small molecule drugs and antibody drugs. Small molecule drugs and antibody drugs targeting G protein-coupled receptors (GPCRs) have different characteristics and advantages [1].
Small molecule drugs are often used to modulate the activity of GPCRs by binding to their orthosteric or allosteric binding sites. They have the advantage of being orally available and can easily penetrate cell membranes to reach their target. Small molecule drugs have a relatively low manufacturing cost and can be produced in large quantities. However, they can have off-target effects and may be less specific to their target receptor, which can result in side effects [2].
In contrast, antibody drugs are large proteins that bind to specific epitopes on GPCRs. They can be highly specific to their target receptor and can be engineered to have a longer half-life in the bloodstream. Antibody drugs are less likely to have off-target effects compared to small molecule drugs, and they can also activate or block downstream signaling pathways. However, they are not orally available and need to be administered through injection or infusion, which can limit their convenience and accessibility [3].
Overall, small molecule drugs and antibody drugs targeting GPCRs have complementary strengths and limitations, and the choice between them depends on the specific therapeutic application and the properties of the targeted receptor.
To validate the efficacy of potential GPCR agonists or antagonists, researchers use various assays, including cell-based assays, radioligand binding assays, and functional assays.
Cell-based assays
Cell-based assays are commonly used to validate potential GPCR agonists or antagonists. These assays involve measuring changes in intracellular signaling pathways in response to ligand binding. Examples of signaling pathways that can be measured include the activation of adenylyl cyclase, the release of intracellular calcium stores, and the activation of MAP kinase pathways [4].
There are several commercial kits available for cell-based assays of GPCR activity. For example, Promega offers the GloSensor cAMP Assay System, which allows for real-time monitoring of cAMP levels in live cells [5]. Similarly, the FLIPR Calcium Assay from Molecular Devices enables high-throughput screening of GPCR activity through the measurement of calcium mobilization [6].